Follow the Data Podcast: A Promising Treatment for COVID-19
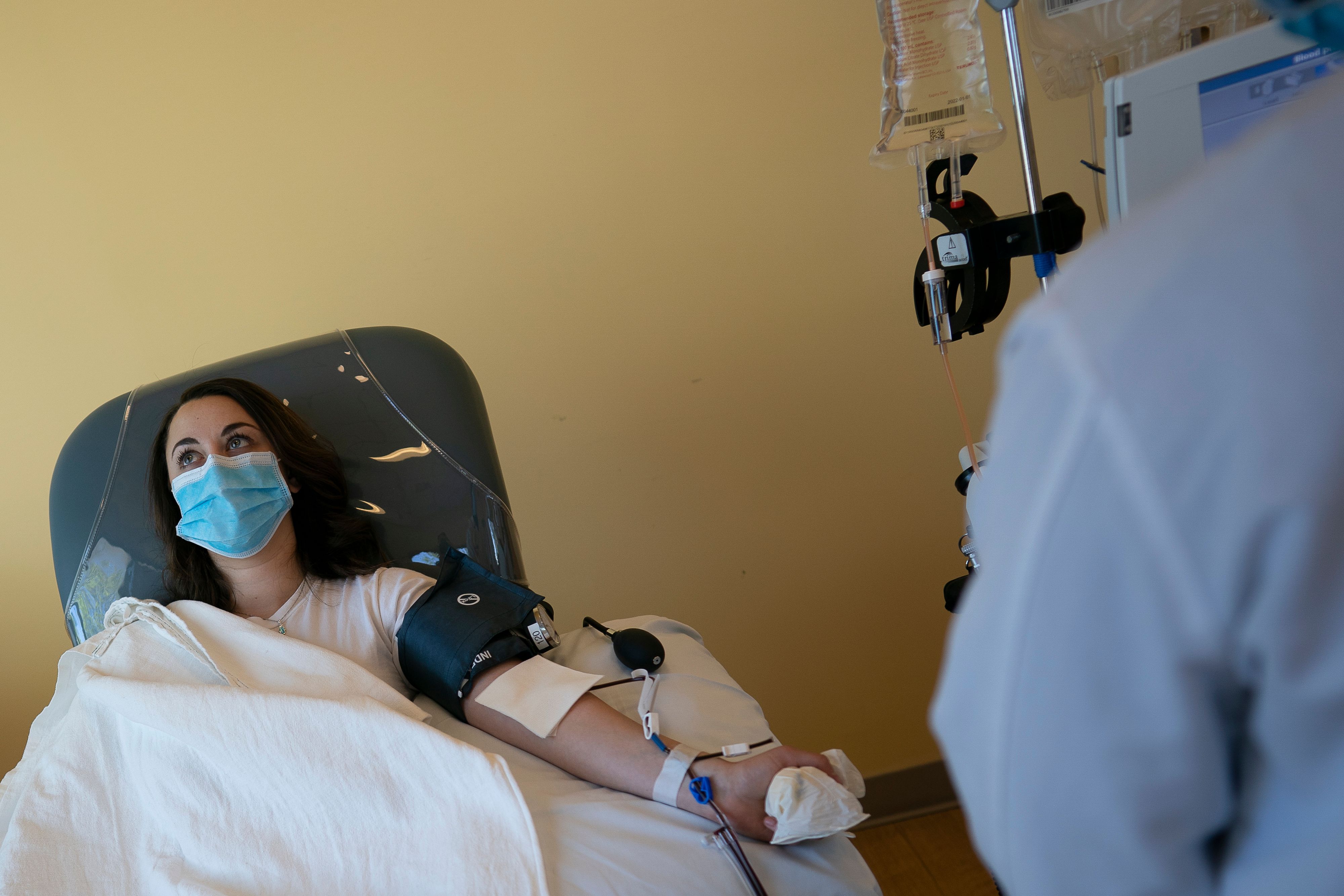
Dr. Arturo Casadevall and colleagues at Johns Hopkins University and around the country have been working around the clock to develop a convalescent serum therapy to treat COVID-19 using blood plasma from recovered patients. If all goes well with the current U.S. trials, thousands of survivors might soon line up to donate their antibody-rich plasma.
Dr. Casadevall, an infectious disease expert and Bloomberg Distinguished Professor, has led a team of physicians and scientists from across the country to establish a network of hospitals and blood banks that can collect and process blood plasma from recovered viral positive patients.
While there are currently no proven drug treatments or vaccines for COVID-19, Dr. Casadevall and his team believe that using blood plasma from fully recovered patients could boost the immune systems of health care responders and first responders.
To support this endeavor, Bloomberg Philanthropies is teaming up with Maryland Governor Larry Hogan and Johns Hopkins to fund the research and teams working on developing this therapy day and night.
In this episode of our series around Bloomberg Philanthropies’ COVID-19 response, Dr. Casadevall joined Dr. Jessica Leighton, from our public health team, to discuss how blood plasma has been used to treat infectious disease outbreaks in the past, what makes blood plasma treatment different from a vaccine, how donating your blood plasma could help your community, and what’s giving researchers hope right now.
You can listen to the podcast and past episodes in the following ways:
- Stream it on SoundCloud.
- Check us out on Spotify.
- Download the episode from Apple Podcasts and be sure to subscribe.
- Listen to us on Stitcher – be sure to rate and review each episode!
- Tune in on Simplecast.
For more from our coronavirus series:
Amanda McClelland, the Senior Vice President of Prevent Epidemics and Resolve to Save Lives at Vital Strategies sat down with Dr. Jennifer Ellis, who works on our public health program, on our most recent episode, “Slowing the Spread of COVID-19 in Africa.” They discuss why it’s important to prioritize slowing the spread of coronavirus in low- and middle-income countries, the importance of the Box It In strategy, how response to the coronavirus has differed from other recent outbreaks, and what’s keeping public health professionals hopeful right now.
“The Cost of Recovery for Our Cities, Part 1,” features Adam Freed, a Principal at Bloomberg Associates, Natasha Rogers, the Chief Operating Officer of the City of Newark, and Brad Gair, a Principal with Witt O’Brien’s, a national emergency management consultancy, for the first episode in a two-part series about how cities can best navigate, access, and deploy federal aid.
Janette Sadik-Khan, a Principal at Bloomberg Associates and Chair of NACTO, sat down with Corinne Kisner, Executive Director of NACTO, and Mark de la Vergne, the Chief of Mobility Innovation for the City of Detroit, to discuss how cities are continuing to run transit systems while keeping their own staffs safe and the challenges coronavirus poses for city transportation departments in The Intersection of COVID-19 and Transportation.
“Behind the Scenes of the Johns Hopkins Coronavirus Map” was adapted from “Public Health On Call,” a podcast from the Johns Hopkins Bloomberg School of Public Health. Dr. Josh Sharfstein, Vice Dean for Public Health Practice and Community Engagement at the Johns Hopkins Bloomberg School of Public Health, joined Beth Blauer, executive director of Centers for Civic Impact at Johns Hopkins, to discuss how the global COVID-19 tracking dashboard was made, what new features have been added, and how data can help individuals and officials make informed decisions for COVID-19 response.
Dr. Josh Sharfstein also appeared on a recent Follow the Data episode entitled “Responding to a Pandemic Crisis.” He sat down with Dr. Jessica Leighton, who works on our Public Health program, to discuss crisis response for public health practice and what makes COVID-19 different from other recent outbreaks.
Megan Sheekey, who leads Strategic Partnerships at Bloomberg Associates, joined Darren Walker, president of the Ford Foundation and one of Bloomberg Philanthropies’ co-funders in the NYC COVID-19 Response & Impact Fund, for an episode entitled “How to Help Nonprofits Hit Hard by COVID-19.” They discussed how the coronavirus pandemic is affecting social services and cultural organizations in New York and the role of foundations in supporting their work.
Dr. Tom Frieden, the President and CEO of Resolve to Save Lives, sat down with Dr. Kelly Henning, who leads the Public Health program at Bloomberg Philanthropies and was the former Director of Epidemiology for the City of New York, for the first episode of our coronavirus series, “World War C – Us Against the Microbe.” They discussed the global response to COVID-19, how our $40 million Coronavirus Global Response Initiative will help prevent or slow the spread of the virus in low- and middle-income countries, and how we can keep our cities running and safe.
Full Transcript:
Katherine Oliver:
Welcome to Follow the Data, I’m your host, Katherine Oliver.
Dr. Arturo Casadevall and colleagues at Johns Hopkins University and around the world have been working around the clock to develop a convalescent serum therapy to treat COVID-19 using blood plasma from recovered patients. If all goes well with the current U.S. trials, thousands of survivors might soon line up to donate their antibody-rich plasma.
Dr. Casadevall, an infectious disease expert and Bloomberg Distinguished Professor, has led a team of physicians and scientists from across the world to establish a network of hospitals and blood banks that can collect and process blood plasma from recovered viral positive patients.
While there are currently no proven drug treatments or vaccines for COVID-19, Dr. Casadevall and his team believe that using blood plasma from fully recovered patients could boost the immune systems of health care responders and first responders.
To support this endeavor, Bloomberg Philanthropies is teaming up with Maryland Governor Larry Hogan and Johns Hopkins to fund the research and teams working on developing this therapy day and night.
In this episode of our series around Bloomberg Philanthropies’ COVID-19 response, Dr. Casadevall joined Dr. Jessica Leighton, from our public health team, to discuss how blood plasma has been used to treat infectious disease outbreaks in the past, what makes blood plasma treatment different from a vaccine, how donating your blood plasma could help your community, and what’s giving researchers hope right now.
Dr. Jessica Leighton:
We are so pleased to have here with us today Dr. Arturo Casadevall, who’s been leading the way on critical antibody research for COVID-19.
Dr. Arturo Casadevall:
Thank you for having me.
Dr. Jessica Leighton:
It sounds like your work has changed a lot since the start of the pandemic. Can you talk to us a little bit about the history to using convalescent plasma?
Dr. Arturo Casadevall:
In 1890, it was discovered that you could transfer immunity with serum. Serum is the old word for plasma. And this led to the first Nobel Prize that was given to Emil von Behring in 1901. That’s how old this is. And that discovery was key because it really helped launch the science of immunology. And the idea that we had in the liquid of our blood materials that would make others immune was a profound discovery in the history of medicine. And then for many years there were no antibiotics. There were no vaccines, there were no antivirals. And then doctors used this during epidemics. It was used in 1918 epidemic, where a retrospective analysis of all they did showed that it reduced mortality. It’s been used in the 1920s, 1930s, whenever there were epidemics of polio, measles, mumps. It was even more recently used in the SARS outbreak in 2003 in Hong Kong. And even as late as 2009, when H1N1 influenza appeared and went around the globe, doctors used convalescent plasma in several places to treat sick patients.
So this is an old therapy, but it rests on an enormously strong foundation of immunological and basic science knowledge. And it has a long history of having been effective. So when you’re facing a situation like COVID-19, where you don’t have vaccines, where you don’t have antivirals, although hopefully we at least have one now, then it became an obvious choice that needed to be deployed.
And the question was, how do you do it? When there was no infrastructure for doing it. And fortunately, because of the rights of self association that we have in this country, free speech, ability to get things done, a large number of people rose to the occasion.
Dr. Jessica Leighton:
Can you explain a little bit more about blood plasma and convalescent plasma, exactly what these terms mean?
Dr. Arturo Casadevall:
Regular plasma can be from anybody. People don’t have to have disease. That’s how we use the term differently. So what we are interested in is in the use of the convalescent plasma. People who recovered. People who have antibodies in their blood, to use that part of the blood, the liquid part, to transfer immunity to individuals who don’t have it. Because once you get the plasma, you have their antibodies, so you have the protection that they have.
So, that can be used for prevention of disease, and can also be used for treatment of disease. Because those antibodies will begin to neutralize the virus that is in the lungs. And the idea is, that as the virus gets neutralized, the body gets a jump start that allows it to begin to repair and begin to get going, such that it can get ahead of the disease.
Dr. Jessica Leighton:
So can the convalescent plasma be used as a vaccine? How is that different from the vaccines that are being developed?
Dr. Arturo Casadevall:
It’s very different. When you get a vaccine, you are the factory. You make the antibodies to a vaccine. You get a flu vaccine, you’re making antibodies to the flu. In the case of convalescent plasma, you’re getting the antibodies from somebody else. So somebody else who had the disease. Somebody else who went first, who’s giving you the gift of their antibodies, to give you immunity or to help treat the infection.
Dr. Jessica Leighton:
Your team has been doing a lot of work on this convalescent plasma. We’ve heard that convalescent plasma is already being used to treat people around the country, and we’ve heard that the treatment seems to be successful. Can you explain what is being done, and how many people have already received this convalescent therapy? And is this enough to assure us that it can work?
Dr. Arturo Casadevall:
It’s a complex question, so let’s try to unpack parts of it. The use in the United States, I can tell you that as of today there are probably about 9,000 people that have been treated. That is a large number of people that have received convalescent plasma. These people have been treated through what is called an investigational new drug that allows doctors to decide when this might be helpful. This is not clinical trials. This is a very large case series. We think that there is information there that we can mine. For example, we think that… I believe very shortly there will be reports that it is safe to be used.
The question of efficacy is going to be harder to piece out, because you are not using a controlled clinical trial. What you’re doing is you’re giving it to people, you’re observing what happens, and you’re comparing it to people who did not receive it. So, that’s always not fraught with potential bias. It is not as the type of information that comes from controlled clinical trials.
We look at them with encouragement, we look at them with optimism, but we don’t look at them as evidence of efficacy. To get evidence of efficacy you really need to analyze the data in a dispassionate way, and conduct randomized clinical trials.
So, it’s been used, but randomized clinical trials are beginning. And they’re beginning… Well, many of them have been done including, for example, the National Health Service in the United Kingdom is beginning a randomized controlled clinical trial. So, I think we’re going to have really good data on efficacy in the next few months, but I would ask people to defer judgment for now, because the kind of information that we have is not the kind of information of which we like to base decisions and give advice.
Dr. Jessica Leighton:
So, you’ve created a large team of physicians and scientists from around the United States to establish this network of hospitals and blood banks that can collect, isolate, and process plasma from COVID-19 survivors, and be used in the randomized controlled trials. Can you tell us a little bit about the research projects that you and your team are doing, and give us some updates about how it’s going?
Dr. Arturo Casadevall:
So, people give me too much credit. This group emerged. It emerged from a collection of friends. And these people are people like Mike Joyner at Mayo, Nigel Paneth at Michigan State University, Liise-anne Pirofski in Einstein. This was a community that began to kind of work together. And they put their ideas, their mind, their resources to solve some of these problems. And I think there is a beautiful story or how without any governmental or institutional organization, physicians and scientists can organize to do something that needed to be done, and to do it in a matter of weeks. Which, even though when you’re in the middle of an epidemic, it feels like everything is moving very slowly, the fact is, that when you look, when you consider what has been done relative to how long clinical trials, or anything gets done clinically, it’s been almost in the blink of an eye.
Dr. Jessica Leighton:
How long do you think it will take until you have some results?
Dr. Arturo Casadevall:
I think we will have results…. I think we’re going to have early results, very shortly. In a matter of weeks. What do I mean by early results? People are already writing up their case series. So these experiences with 9,000 people will come out, and they will give us a hint as to efficacy. And they will give us some pretty good information on safety.
But, the question that I think you’re asking is, how are you going to know for sure? It’s going to be when the clinical trials are completed. And that is going to take a few months. And, because you’re going to have to set up control groups, you’re going to have to set up patients, you’re going to have to analyze the data, and the biggest determinate on how rapidly we know is how rapidly we accrue patients. If you’re able to do hundreds of patients in a couple of weeks, we’ll know by June. If you’re going to take you two months to do a couple hundred patients, will know by July.
Dr. Jessica Leighton:
So, you need to get plasma from people who’ve already had COVID-19. What would you say to people who are considering donating their blood plasma if they’ve been infected before? If one person was to donate their serum, in theory, also, how many other people could they help?
Dr. Arturo Casadevall:
So, the math is good. One person can at least help two, and possibly three. If you had COVID-19 we urge you to donate your plasma. Today there is a great need of plasma. Not everyone who needs it is getting it. But where there is a great need of plasma for tomorrow, because the pharmaceutical industry is organizing to create what are known as gamma globulins. These are preparations of antibody. They are better in many ways than plasma. Because, when you get plasma, plasma units can differ a lot. You can have a person that has a lot of antibodies. You could have a person that has less antibodies. Whereas, if you could take the antibody out of the plasma, and create a formulation that you put in a vial that is standardized, that’s what gamma globulin is known. That will be a superior product, and we think that that’s going to be available late summer, early fall.
So, please donate plasma because your plasma can be used to help people directly today, or it can be used to get a [glitchy] product that will help people tomorrow.
Dr. Jessica Leighton:
We have heard that there are different kinds of tests being done for COVID-19, and you could get a test for antibodies, but you could also get a test for infection. Is that different?
Dr. Arturo Casadevall:
Yeah, very different. So the tests that are done with the swabs, what they do is they detect the virus. The detect the viral nucleic acids. And, they tell you that the virus is there. But, if you recover, you clear the virus. And, how do you know whether you recovered or not? Then your test will be negative. Well, then the next test that is very helpful is an antibody test. Because the antibody test remembers that you have been infected, because you know have antibodies through the virus in the blood.
These two are very different. One of them tells you whether you’re infected and whether you have a chance of infecting others, and the other one tells you whether you have seen the virus, perhaps eliminated it, and are now immune.
Dr. Jessica Leighton:
As an immunologist, is there any evidence that, if you’ve been infected with COVID-19 you can be infected with it again?
Dr. Arturo Casadevall:
So, I’ve seen those reports, but I will tell you… They’ve been in the news, and I will tell you that, those of us are deferring judgment. In part because we know that this coronavirus makes neutralizing antibodies. When you have neutralizing antibodies to a virus, you’re usually immune. So, for me to really believe the case reports, I would have to see a very well done study, by somebody who’s shown to have COVID-19, clear the infection, and then have COVID-19 again. A lot of these case reports are unclear on whether people had it in the first place. Because, as you know, testing has not been as available as we would like.
So, I would defer judgment on that. But it is my belief, today on May 11, based on what I know, that individuals who make neutralizing antibodies to this virus, probably are protected. Or, at least they’re protected for a while. We don’t know if this is going to be like measles, that gives you lifelong immunity, or it’s going to be like influenza, where you have to get a shot every year. But, at least, in the short term, these individuals who recover have in their blood antibodies that kill the virus. And that, to me, means a high level of protection.
Dr. Jessica Leighton:
So, it sounds like a lot of people need to have had COVID-19 to prevent others from getting it. We hear the concept of herd immunity. Can you explain that? And, if we don’t have a vaccine, is there any chance that we could achieve herd immunity?
Dr. Arturo Casadevall:
Well, let’s just take… What is herd immunity? Herd immunity means that enough people have been infected that the virus can’t jump very well from person to person because when it makes the jump it’s going to run in to an immune person. But, that usually requires about 70 percent of the population to have immunity. It depends, and sometimes it’s a little higher, sometimes it’s a little lower, but let’s just take the 70 percent number. Seventy percent will require a lot of people to get sick, and it will have a lot of people die.
So, I think that, if we didn’t have modern medicine, if this had happened 100 years ago, we would have gotten herd immunity because this would have basically gone through the population. But, hopefully we can continue to reduce the likelihood of transmission by social distancing until a vaccine is found. When a vaccine is found, I think that we then will be able to vaccinate a large percentage of the population, and then you’ll have herd immunity. Because the virus, when it jumps, it’s likely to jump into hosts that can’t replicate it.
So, I’m a big supporter of the current social distancing guidelines. I will give you just an anecdote to think about. If you got sick on March 1, or you get sick today, your chances of being asymptomatic, or being mildly sick, or being very sick are roughly the same. But, in March 1 you did not have remdesivir. On March 1 you did not have plasma. You have it two months later.
If we continue to flatten the curve, those who get infected in the summer or early fall will have a lot more options. And, if we figure out how to use these options well, we can reduce mortality, and we can begin to take some of the terror of this viral infection away.
Dr. Jessica Leighton:
It sounds like you’re feeling really optimistic about where things will go. What gives you most hope as it relates to the global response to the coronavirus?
Dr. Arturo Casadevall:
Well, this virus has jumped into a species that has a biomedical research establishment. That is able to respond very rapidly, because society spends its money to create it. If you think about it, today our aircraft carriers can’t help us, our bombers can’t help us. Society’s line of defense is running through the biomedical research establishment. And this is a research establishment that, in five months, has identified the virus, has learned tremendous about it, and is rapidly trying to find new ways to treat it.
So, we have learned already that the virus elicits a neutralizing antibody. We have learned that there are 80 or so vaccines in development. We have learned that monoclonals are being developed. We have learned that antivirals are being screened and developed. So, I am very optimistic that humanity will beat this coronavirus.
How long it will take us? It’s going to take us some time. Simply because with vaccines you cannot rush development. Think about it, if you get a vaccine it still takes a few weeks to get an antibody response.
Imagine what it would have been like to deal with this without scientific research knowledge, or modern medicine, or respirators, or the ability to provide supplemental oxygen. This is the way they dealt with in 1918. In 1918 they didn’t have any of this. People who got sick, unfortunately, were on their own. Today we can do a lot more.
And 1918 may seem 100, 102 years away, however, there are people alive today who were alive then. This is four generations. This is the tremendous progress that we have made. And I believe that this calamity is going to lead to new work that will make us even better prepared. And if the history of science is any indication, some of the things that we learn against coronavirus will be applicable to other diseases. So, yes, I’m very optimistic.
Dr. Jessica Leighton:
It’s great to hear your optimism, and we really want to thank you for joining us today. We look forward to speaking to you again and hearing the progress of your research. Thank you very much, Dr. Casadevall.
Dr. Arturo Casadevall:
Thank you.
Katherine Oliver:
We hope you enjoyed this episode of Follow the Data.
If you have recovered from COVID-19 and are now symptom-free, you can learn more and discover where to donate blood plasma at ccpp19.org. If you live in the Maryland, DC and Virginia area, consider donating plasma at Johns Hopkins Medicine. Email JHUCOVIDplasma@jhmi.edu to coordinate donations. It could save your neighbor’s life.
Many thanks to Dr. Jessica Leighton and Dr. Arturo Casadevall for joining us. If you haven’t already, be sure to subscribe to Follow the Data podcast and tell your friends to subscribe as well!
This episode was created by Devin Alessio, Ivy Li, Amie Juhn, and Lauren Nolan.
As our founder Mike Bloomberg says, if you can’t measure it, you can’t manage it. So until next time, keep following the data.
I’m Katherine Oliver, thanks for listening.